会议主题
会议议题包括,Certara公司概况、Phoenix平台概况、中国用户代表发言、Phoenix软件未来开发计划、Phoenix产品案例演示等环节,Certara公司将持续倾听用户的声音,不断的改进他们的软件,本次会议所使用的演讲稿件请点击以下链接下载。
点击即可下载:用户会演讲PPT
互动问答
会议过程中,Certara讲师与观众进行了一系列的互动问答,现已进行了整理内容如下:
第一天:
Audience question 1:
观众提问1:
Could you share the lecturer's PPT with me?
可以将讲师的PPT分享给我么?
Tomoo Answer:
Tomoo回答:
Yes.
可以。
Audience question 2:
观众提问2:
There is a "Dose" worksheet in the data. How do you link the "Dose" worksheet to the NCA object?
数据中有一个“Dose”工作表。如何将“Dose”工作表链接到NCA对象的“Dosing”?
Cindy Answer:
Cindy回答:
Go to setup, and then dosing. The user can directly drag the dose datasheet to the right pane and then do the mapping.
在NCA 对象中,导航至“设置(Setup)”标签页,然后到“剂量(dosing)”表格。用户可以直接将左侧对象浏览器中的“剂量(dose)”工作表拖到右侧窗格中NCA 对象“剂量(dosing)”表格中,之后进行映射即可。
Audience question 3:
观众提问3:
How to get the average pharmacokinetic curve?
如何在Phoenix中绘制平均药时曲线。
Cindy Answer:
Cindy回答:
To plot the average PK curve, send the dataset to NCA and Toolbox à Descriptive Stats. In Descriptive Stats, Sort by Nominal Time, and any other required Sort variables (e.g. Treatment, Analyte, Cohort), and map Concentration as the Mean. Execute Descriptive Stats. Then send the Descriptive Stats result to an XY plot, map Nominal Time to X, Mean to Y, and SD or SE as Error variable. Then Execute the XY plot.
要绘制平均PK曲线,请鼠标右击数据集,在右键菜单中依次选择“Send to(发送到)”→“NCA and Toolbox(NCA和工具箱)” →“Descriptive Stats(描述性统计)”。在“Descriptive Stats(描述性统计)”对象中,将“Nominal Time (标称时间)”映射至“Sort(排序)”字段,将其他所有必需的“Sort(排序)”变量(例如“Treatment(处理)”,“Analyte (分析物)”,“Cohort”),然后将“Concentration (浓度)”映射至“Mean (平均)”字段。然后执行“Descriptive Stats(描述性统计)”对象。之后将“Descriptive Stats(描述性统计)”结果中的工作表发送到“XY plot(散点图)”对象,将“Nominal Time (标称时间)”映射到”X”字段,将均值映射至”Y”字段,并将“SD”或“SE”映射至“Error bar”字段,最后执行“XY plot(散点图)”对象。
Audience question 4:
观众提问4:
How to accurately calculate the half-life of Enterohepatic Recirculation?
如何准确计算肠肝循环的半衰期?
Cindy Answer:
Cindy回答:
For the enterohepatic circulation, you need to make sure the terminal phase is fully captured. If enterohepatic circulation is noticed for a compound, then the collection time will be extended correspondingly to make sure the terminal phase is reached.
对于肠肝循环,您需要确保完全捕获终末段药时曲线。 如果发现化合物有肝肠循环,则样品收集时间将相应延长,以确保达到终末段。
第二天:
Audience question 1:
观众提问1:
Why is the point estimate of GMR different in ABE and RSABE?
为什么在ABE和RSABE中GMR的点估计不同?
Chris Answer:
Chris 回答:
The point estimate should generally be the same for ABE and RSABE. The exception is the example that I showed with some missing data. If a subject is included in ABE, but excluded from RSABE, the point estimates will be different.
ABE和RSABE的点估计值通常应该相同。我所显示的示例缺少一些数据,这是一个例外。如果某个个体包含在ABE中,但未包含在RSABE中,则点估计将有所不同。
For the NTID template, a full replicate (2 treatments by 4 periods) is required. A subject is included in ABE point estimate if data is available for at least one period. A subject is excluded from RSABE point estimate if data is missing for any periods.
对于NTID模板,需要完整复制(2个处理×4个周期)。 如果数据至少有一个周期可用,则将一个个体包括在ABE点估计中。 如果在任何时期内都缺少数据,则将该对象从RSABE点估计中排除。
Audience question 2:
观众提问2:
Hi Kevin, what's the time interval for updating the CDISC terms in WinNonlin?
嗨,Kevin,在WinNonlin中更新CDISC术语的时间间隔是多少?
Kevin Answer:
Kevin 回答:
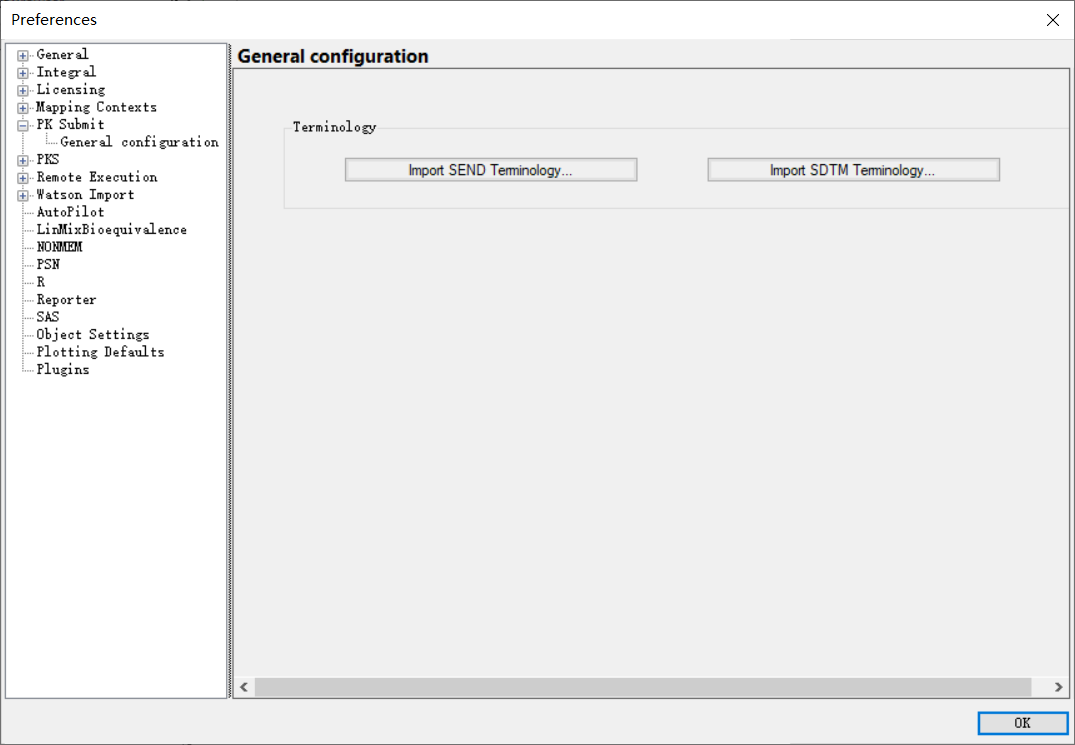
Controlled terminology (updated every 3 months by CDISC) can be updated any time by the user by simply uploading the relevant Excel workbook published by CDISC into the system through Edit Preferences.
用户可以通过菜单“编辑(Edit)”→ “项(Preferences)”→ “PK Submit” → “一般配置(General configuration)”将CDISC发布的相关Excel工作簿上传到Phoenix系统中,通过这样简单地操作,来随时更新受控术语(由CDISC每3个月更新一次)。
Audience question 3:
观众提问3:
What is the difference between POWER_TOST and POWER_80_20?
POWER_TOST和POWER_80_20有什么区别?
Chris Answer:
Chris 回答:
POWER_TOST is Power computed by the “Two One-Sided T-test” rule, as described in (Phillips, K. F. (1990), or Diletti, E., Hauschke, D., and Steinijans, V. W. (1991):
POWER_TOST是通过“双单侧T检验”规则计算的把握度,如(Phillips,K. F.(1990)或Diletti,E.,Hauschke,D.和Steinijans,V. W.(1991)中所述:
Power_TOST = p( -tcrit, df, t2) - p( tcrit, df, t1)
The Power_TOST procedure is more likely to be of use with simulated data, such as when planning for sample size, rather than for drawing conclusions for observed data.
Power_TOST过程更可能与模拟数据一起使用,例如在计划样本大小时,而不是为观察到的数据得出结论时。
Power_80_20 is Power computed by the 80/20 rule, as described in pg. 142–143 of Chow and Liu (2000).
Power_80_20是通过80/20规则计算的把握度,如Chow and Liu(2000)第142–143页中所述。
Power_80_20 is approximated by 1 - [p(T>t1) - p(T>t2)]
Power_80_20近似为1-[p(T> t1)-p(T> t2)]
where T has a central t distribution with df=Diff_DF.
其中T具有df = Diff_DF的中心t分布。
More information, including special cases for each rule, is provided in the Phoenix Online Help and the associated pdf.
Phoenix在线帮助系统和相关的pdf中提供了更多信息,包括每种规则的特殊情况。
第三天:
Audience question 1:
观众提问1:
How to determine what kind of PH value, dissolution curve under stirring rate can be used to establish IVIVC model.
如何确定哪种PH值,搅拌速率下的溶出曲线可用于建立IVIVC模型。
Chris Answer:
Chris回答:
An aim of in vitro experiments is to maximize solubility of the compound under in vitro conditions that match the pH of the proportional absorption site where one estimates ka (e.g. in the small intestine).
体外实验的目的是在体外条件下较大限度地提高化合物的溶解度,可以与比例吸收位点的pH值相吻合,在该位置可以估算出ka(例如在小肠中)。
A good review of this topic is provided here:
Int J Pharm. 2011 Oct 10; 418(1): 142–148.
这里提供了对该主题的很好的评论:
Int J Pharm。2011年10月10日;418(1):142–148。
Audience question 2:
观众提问2:
How to choose the weight of in vivo data fitting.
如何选择体内数据拟合的权重。
Answer:
回答:
Chris:
For PK compartment models, a standard workflow is to start with uniform weighting. After the first run, view residual plots such as CWRES vs Time and CWRES vs PRED. For an unbiased model, one looks for random scatter of the residuals. If a pattern such as fan-shape or U-shape is detected, consider using a different weighting scheme in the next model run. For PK data, a multiplicative (1/Yhat squared) weighting scheme is often found to be optimal. This topic is discussed in detail in the following reference:
对于PK房室模型,标准的工作流程是从“均匀(uniform)”(译者注:即相当于我们所说的不加权)加权开始。 第一次运行后,查看残差图,例如CWRES vs Time和CWRES vs PRED。 对于无偏模型,人们会寻找残差的随机散布。 如果检测到诸如扇形或U形之类的模式,请考虑在下一个模型运行中使用其他加权方案。 对于PK数据,通常发现乘法(1 / Yhat平方)加权方案是比较好的。 以下参考文献详细讨论了该主题:
Gabrielsson and Weiner (2015) Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications, 5th ed. Swedish Pharmaceutical Press, Stockholm.
Gabrielsson和Weiner(2015)药代动力学和药效动力学数据分析:概念和应用,第5版。 瑞典医药出版社,斯德哥尔摩。
礼品与奖品
在会议的进行过程中,和会议结束后,源资科技和Certara也一起联合为大家带来了丰富的礼品。
包括:
注册与转发礼品: “Phoenix WinNonlin基础培训班” 线下培训“200元抵扣优惠券”
出席参会抽奖礼品:10元现金洪浩,100元京东购物卡,200元京东购物卡
连续出席参会抽奖礼品:“Phoenix WinNonlin基础培训班” 线下培训“1700元抵扣优惠券”,Phoenix WinNonlin365天免费试用。
以上奖品都已经抽取完毕,让我一起祝贺获奖的小伙伴~
Phoenix产品试用
会议结束后我们也开放了Phoenix产品试用申请通道,已收到大量用户的试用申请,Certara公司正在对这些试用申请进行审核与处理,申请试用的小伙伴还需要耐心等待会哦
相关链接: Phoenix WinNonlin培训课程
感谢所有参与本次会议小伙伴,让我们下次“Phoenix产品用户会”再见~!



